5.1 Introduction
Who reads textbooks? Students, of course. But also their professors and other professionals, including both specialists, who need to keep up with the latest developments, and others who want to maintain a comprehensive picture of their discipline. Not least, textbooks can be a treasure trove for historians: Textbooks give us a snapshot of the state of a discipline in a particular time and place, and from a particular point of view.1
Here I will examine Fritz Reiche’s 1921 quantum theory textbook (Reiche 1921a).2 At its publication, barely 20 years had passed since
Fritz Reiche was born in 1883 and earned his Ph.D. in 1907, as one of
In 1913, Reiche was back in Berlin and, with Planck’s support, qualified as a Privatdozent (instructor) at the University of Berlin. He was an assistant to
5.2 Fritz Reiche and Die Naturwissenschaften
The story of Reiche’s textbook begins in 1913, when Arnold Berliner persuaded Ferdinand Springer to establish a new journal, Die Naturwissenschaften (The Natural Sciences), which, like Nature in Britain and Science in the United States, would report on new developments in all of the natural sciences for all scientists. As Berliner and his co-editor Curt Thesing put it in an editorial in the very first issue,
The rapidly progressing specialization in all branches of research in the natural sciences [Naturforschung] makes it difficult for the individual to become informed about even neighboring domains. It is almost impossible for him to become acquainted with more distant ones […]. “Die Naturwissenschaften” is determined to fill this gap.
As his friend
insisted that every article in Die Naturwissenschaften should be written in such a way that his “simple mind” could understand it. How few of the contributions proved up to the high standard which he set, and how lively was the ensuing correspondence. (Born 1942, 285); quoted in (Stöltzner 2003, 172)
Another friend,
Berliner addressed himself mainly to the then young generation of men of science. Much of the success of the journal was due to Berliner’s vivid personality, his close contact with the majority of young physicists and mathematicians and his initiative in formulating the subject of articles he wanted written for his journal. Thus Die Naturwissenschaften became a mirror reflecting the development of science during 1913–30. (Ewald 1942, 284); quoted in (Stöltzner 2003, 171)
Reiche began writing for Die Naturwissenschaften in the first (1913) volume. He may well have come to Berliner’s attention via
If someone, standing at the window of an evening, looked through a fogged or frost-covered window pane by the light of a street lantern, he would see the light surrounded by colored rings, in which he would easily recognize the colors of the rainbow. (Reiche 1913b, 1193)
By stages, he drew his readers into a careful, detailed, but non-technical discussion of wave motion, diffraction, and interference that ended with a description of
This piece was not Reiche’s first contribution. Six months earlier, in June 1913, Reiche published a nine-page, two-part article on quantum theory, intended to summarize the deliberations of the
It has become necessary to introduce a discontinuity into our physical ideas, an element that can change only in jumps, whose existence we had not suspected until a few years ago. (Reiche 1913a, 572)
With this article, Reiche showed his ability to present at an introductory level what even in 1913 was a complex, many-faceted subject. And although another four years would pass before he began to publish his own research on quantum theory, it is evident that by 1913 he already had an encyclopedic knowledge of the subject. More generally, we see that quantum theory was sufficiently established to merit several articles for non-specialist readers in Die Naturwissenschaften.
5.3 Interlude: The Quantum Underground
Between 1913 and 1936, Reiche published some twenty book reviews in Die Naturwissenschaften, the majority during the teens and early twenties, and many of them on quantum theory. Most of these reviews are unsurprising. For example, he reviewed
But in 1914 and 1915, Reiche also reviewed two books by
That final chapter had even earlier roots. It had appeared late in 1912, in the Proceedings of the Karlsruhe Natural Scientific Society, with the more descriptive title “On the Radiation Law, the Action Quantum, and
In 1914,
In that same year, the British physicist
There are a few themes common to all four of these books:
1All three authors were skeptical of
2All three showed a marked preference for Planck’s “second theory,” in which oscillators absorbed energy continuously but emitted energy quanta.
3All three emphasized the photoelectric effect and promoted quantum (albeit not light quantum) explanations. Historians often (and correctly) criticize modern textbooks for emphasizing the photoelectric effect, sometimes at the expense of the more fundamental aspects of
4All three made use of a February 1911 paper by
When
5.4 Reiche’s Textbook and the State of Quantum Theory in 1921
Volume 6, Number 17 of Die Naturwissenschaften appeared in April 1918, just as Germany was launching the offensive on the Western Front that it hoped would bring victory in the Great War (Keegan 1998, chap. 10). The issue was devoted to a Festschrift celebrating
The book appears to have sold well. Inexpensive copies are still widely available on the used book market. The English edition may have been especially popular; it went through three printings, the second two in 1924 and 1930. For the 1930 edition,
As in his earlier Naturwissenschaften pieces, Reiche’s prose was clear and forceful; I give a few examples later. The level remained introductory. The book is, on the whole, historically accurate; even today, one can get from it a good picture—from Reiche’s point of view, to be sure—of the state of quantum theory around 1920.
Reiche’s 1914 review of
As happy as we are to welcome such an introduction, it nevertheless appears to me difficult to find the correct boundary between a strong mathematical line of argument on the one hand, and arguments that are, as much as possible, mathematics-free and still persuasive on the other. It is a well known difficulty with which all popular accounts must struggle. (Reiche 1915)
By 1921 Reiche had found a solution to this dilemma. His main text was about 160 pages (125 pages in the English translation), but included an additional 25 pages of endnotes, many of them extending and deepening the treatment in the text. Readers seeking only an introduction could confine themselves to the text. Advanced readers would find more detailed and mathematical discussions, as well as citations to the research literature, in the notes.19
Reiche’s coverage was comprehensive, as one can see from the following chapter outline. Very few topics in quantum theory went unmentioned, and on unsettled questions, Reiche usually gave a careful summary but refrained from taking sides. Note especially the emphasis given in Chapter V to molecular topics—the specific heat of hydrogen and infrared absorption spectra— which, in fact, had provided some of the earliest experimental support for quantum theory (Gearhart 2010). Reiche’s treatment reminds us that the scope of quantum theory extended far beyond black-body radiation, the specific heats of solids, and atomic spectra:
Chapters I, II: Black-body radiation, including experiments; the Stefan-Boltzmann law; Wien’s law;
Chapter III:
Chapter IV:
Chapter V: Gas theory, including the rotational specific heat of hydrogen; infrared molecular absorption spectra; theories of degenerate gases; chemical (or entropy) constant.
Chapter VI: Atomic spectra, including the
Chapter VII: Quantum theory of
Chapter VIII: Molecular models; dispersion; further discussion of molecular spectra.
Reiche’s treatment of
Here at the entry door to the new land yawns a gulf, which either […] must be bridged by a compromise, or else can be ruthlessly widened by a break with tradition.Einstein felt compelled to take the latter radical step. He proposed the hypothesis that the energy quanta not only played a role in the interaction between radiation and matter […] but that radiation also […] had a quantum structure. (Reiche 1921a, chap. III, § 2)
There followed a several-page, detailed description of Einstein’s arguments, including his 1909 proposal of the wave-particle duality based on energy and moment fluctuations, as well as a careful summary of the experimental evidence. And later on, in a discussion of
Einstein […] was led to the remarkable conclusion that the radiation of Bohr atoms cannot take place in spherical waves, […] but that the process of emission must have a particular direction, like a shot from a cannon. One cannot fail to recognize that the picture of a quantum structure of radiation is thereby brought within easy reach. (Reiche 1921a, chap. VI, § 11)
So far, it sounds as if Reiche were a strong proponent of light quanta. But immediately, he proceeded to give the opposing view:
With all these successes that the light quantum hypothesis can offer, one must still keep clearly in mind that this radical idea […] can be brought into agreement with classical theory only with difficulty. But since interference and diffraction phenomena […] are best reproduced by the wave theory, and the light quantum leads to almost insuperable difficulties, it is understandable that only a few researchers could bring themselves to sanction so drastic a change […].M. Planck defended (and defends to this day) this cautious and conservative standpoint, in which he located the significance of the quantum in matter—or at the least, in the interaction between matter and radiation. (Reiche 1921a, chap. III, § 6)
Reiche went on to describe Planck’s “second theory.” Beginning in 1911, Planck set off in a new direction, motivated by a 1910 calculation of 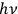 when they cross the boundaries of finite cells of size
when they cross the boundaries of finite cells of size
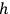 in the two-dimensional phase space that he had first introduced in his 1906 Lectures22 (Planck 1906, § 150); see also (Kuhn 1978; Gearhart 2002). Planck’s second theory was thus an alternative to
in the two-dimensional phase space that he had first introduced in his 1906 Lectures22 (Planck 1906, § 150); see also (Kuhn 1978; Gearhart 2002). Planck’s second theory was thus an alternative to
In the end, Reiche left the question open, and in his brief conclusion to the book, pointed to the provisional and downright murky character of the new quantum theory:
If we now survey the whole structure, as it stands before us, from its foundations to the highest story, we cannot avoid a feeling of admiration: admiration for the few who clear-sightedly recognized the necessity for the new doctrine and fought against tradition, thus laying the foundation for the astonishing successes that have sprung from the quantum theory in so short a time.
Nonetheless, no one who studies the quantum theory will be spared bitter disappointment. For we must admit that, in spite of a comprehensive formulation of quantum rules, we have not come one step nearer to understanding the heart of the matter […].
The decision has not yet been made, as to whether, asPlanck’s first theory requires, only quantum-allowed states exist […], or whether, according to Planck’s second version, intermediate states are also possible. We are still completely in the dark about the details of the absorption and emission process, and do not in the least understand why the energy quanta ejected explosively as radiation should form themselves into the trains of waves which we observe far away from the atom. Is radiation really propagated in the manner claimed by classical wave theory, or does it also have a quantum character?
Over all these problems there hovers at the present time a mysterious obscurity. (Reiche 1921a, chap. IX)
5.5 Reviews
Reiche’s book was widely and positively reviewed. The British journal Nature reviewed both the German edition and the English translation (Anonymous 1922; Allen 1923).23 The first of these reviews, which observed that the book “is an admirable account of the whole field of quantum theory,” went on to take a jab at the Germans:
the literature is very predominantly German, and it is customary in Germany to permit the publication of much more speculative ideas than is usual in other countries. The great merit of the present book is that it brings together all the threads of the argument and criticizes them, so that a just view can be obtained of the whole theory.
The anonymous reviewer went on to suggest that, if anything, Reiche had been too even handed:24
There is little to criticize in such a fair account of the whole theory, but we may venture to say that the author is perhaps inclined to favor Planck’s second hypothesis rather more than would the general consensus of present opinion. [… N]either ofPlanck’s hypotheses has yet been made to cover the facts in a really convincing manner.
In the second review (Allen 1923, 280), the spectroscopist H. Stanley Allen sympathized with Reiche’s reluctance to take sides in the matter of light quanta, writing that “the extraordinary problem […] has been well put by
In many ways, the transference of energy suggests the return to Newton’s corpuscular theory. But the wave theory is too firmly established to be displaced from the ground that it occupies. We are obliged to use each theory as occasion demands, and to wait for further knowledge as to how it may be possible that both should be true at the same time.25 (Bragg 1921, 374)
The Mathematical Gazette noted the introductory character of the book, saying that “Several experimental physicists have found it to be very instructive” (Piaggio 1923). The Bulletin of the American Mathematical Society added:
The author disclaims any intention of writing a systematic textbook, yet he has produced as systematic a text as exists on the subject, and a very readable one. […] The book should not be used as a substitute forPlanck’s Heat Radiation or Sommerfeld’s magnificent Atombau und Spektrallinien, but as an introduction […] with which one may physically orient oneself before taking up more complex discussions.27 (Phillips 1922)
The American physicist
this little book contains a systematic and compact review of the quantum theory […]. Errors of fact or translation are scarce […]. In the absence of a preface one cannot be sure for what class of readers the book was intended by the author. It is quite unsuited for use by a class and would hardly do even as a first introduction for a more experienced reader. It will, however, serve admirably as a good index to the quantum theory as it existed four or five years ago. (Kennard 1924)
Not even historians of science were left out.
Reiche’s book will be very useful not simply to the student of physics, but also to the historian of modern science […].
The theory of quanta is still full of mystery; suggestive and useful as it is, one can but feel that we have not yet reached the bottom of it. (Sarton 1921)
5.6 Who Read Reiche’s Book?
So Reiche’s book was favorably reviewed. In all likelihood it sold well. It surely became widely known. But who bought it, and how was it used? It is not so difficult to discover where quantum theory was being taught, and often, who was teaching it.28 But what textbooks were used in these courses? Or were textbooks used at all? Most early quantum theory courses were for advanced students, whose instructors often relied on their own lecture notes.29 Of course, students could and undoubtedly did seek out whatever supplementary material they could lay their hands on. But I have found it difficult to uncover more than anecdotal evidence. For example, the Italian physicist
Besides what was taught at school, I studied some physics books […] on my own. I still have Glazebrook’s Light,Maxwell’s Theory of Heat, and above all Reiche’s Die Quantentheorie, which greatly impressed me […]. Usually I read these books at school during boring classes. (Segrè 1993, 33)
Similarly, the Japanese theorist
My interest in physics gradually deepened, and I became dissatisfied with the physics I learned in school […]. One day I found a book entitled Quantum Theory, written by the German physicist Fritz Reiche and translated into English, and I bought it. With my knowledge of only high school physics, it was hard to understand […].
Still, I could feel that theoretical physics was in a state of confusion, with discrepancies to be seen everywhere […].
Never, in my life, have I received greater stimulation or greater encouragement from a single book than I did from that one. (Yukawa 1982, 145–147)
A third example suggests another audience for Reiche’s book. One of my own copies of the English translation has the name
A final example comes from
In 1949 I was completing course work for a Ph.D. degree at New York University (NYU) but needed one additional course in statistical mechanics as a degree requirement. The course was given by a diminutive professor with a slight German accent whose name was Fritz Reiche. This course turned out to be the most memorable one I was ever to take at NYU […]. The clarity, the seeming simplicity of the concepts […] succeeded in transmitting to the listener the impression that he or she was able to follow deeply and with brilliant clarity the true essence of statistical mechanics […].
When reading Reiche’s book I discovered, not to my surprise, that it had precisely the same flavor that I recalled from Reiche’s lectures at NYU […]. It remains one of the most accessible, and substantive textbooks I have ever read. (Bederson 2005, 453 and 458)
Reiche remained a teacher throughout his life. His papers are on file at the American Institute of Physics, Niels Bohr Library, in College Park, Maryland. There I came across a handwritten manuscript of a modern physics text that seems to date from the mid-1930s (judging from the material on nuclear physics), when Reiche was back in Berlin after being dismissed from his professorship in Breslau. It is a sad document to read. Reiche must have known that it could never have been published in Germany. It serves to remind us, as we study the exciting days of early quantum theory, that our actors were players on a wider stage. It is a side of this history that we do well to keep in mind.
Abbreviations and Archives
| AHQP | Archive for History of Quantum Physics. American Philosophical Society, Philadelphia |
References
Allen, Herbert S. (1923). The Development of the Quantum Theory. Nature 111: 279-281
Anonymous (1922). Review of . Nature 109: 234-235
Bederson, Benjamin (2005). Fritz Reiche and the Emergency Committee in Aid of Displaced Foreign Scholars. Physics in Perspective 7: 453-472
Berliner, Arnold, Curt Thesing (1913). Zur Einführung. Die Naturwissenschaften
Born, Max (1913). Die Theorie der Wärmestrahlung und die Quantenhypothese. Die Naturwissenschaften 1: 499-504
- (1942). Dr. Arnold Berliner. Nature 150: 284-285
- (1978). My Life: Recollections of a Nobel Laureate. New York: Scribner.
Bragg, William (1921). Aether Waves and Electrons. Nature 107: 374
- (1922). Electrons and Ether Waves. Scientific Monthly 14: 153-160
Cahan, David (1989). An Institute for an Empire: The Physikalisch-Technische Reichsanstalt, 1871–1918. Cambridge: Cambridge University Press.
Duncan, Anthony, Michel Janssen (2007). On the Verge of . Archive for History of Exact Sciences 61: 553-624
Ewald, Peter P. (1942). Dr. Arnold Berliner. Nature 150: 284
Gearhart, Clayton A. (2002). Planck, the Quantum, and the Historians. Physics in Perspective 4: 170-215
- (2010). “Astonishing Successes” and “Bitter Disappointment”: The Specific Heat of Hydrogen in Quantum Theory. Archive for History of Exact Sciences 64: 113-202
Holborn, Ludwig, Siegfried Valentiner (1907). Eine Vergleichung der optischen Temperaturskale mit dem Stickstoffthermometer bis 1600. Annalen der Physik 22: 1-48
Jenkin, John (1993). Brose, Henry Herman Leopold Adolph (1890–1965). In: Australian Dictionary of Biography Melbourne: Melbourne University Press 269-270
Jensen, Christian (1914). Hermann Sieveking. Die Naturwissenschaften 2: 977-979
Jungnickel, Christa, Russell McCormmach (1986). Intellectual Mastery of Nature 2 Volumes. Chicago: The University of Chicago Press.
Keegan, John (1998). The First World War. New York: Knopf.
Kennard, Earle H. (1924). Review of . The American Mathematical Monthly 31: 450
Knudsen, Ole (2001). O. W. Richardson and the Electron Theory of Matter, 1901–1916. In: Histories of the Electron: The Birth of Microphysics Ed. by Jed Z. Buchwald, Andrew Warwick. Cambridge, MA: The MIT Press 227-254
Kormos Barkan, Diana (1999). Walther Nernst and the Transition to Modern Physical Science. Cambridge: Cambridge University Press.
Kuhn, Thomas S. (1978). Black-body Theory and the Quantum Discontinuity, 1894-1912. New York: Oxford University Press.
Landa, Edward R., John R. Nimmo (2003). The Life and Scientific Contributions of Lyman J. Briggs. Soil Science Society of America Journal 67: 681-693
Lorentz, Hendrik A. (1910). Alte und neue Fragen der Physik. Physikalische Zeitschrift 11: 1234-1257
Müller, Georg (2005) Historie der Technischen Universität Clausthal.
- (2007). Technische Universität Clausthal: Abriss ihrer historischen Entwicklung. Clausthal: Universitätsbibliothek.
Myers, Peter B., Johanna Levelt Sengers (1999). Lyman James Briggs, 1874–1963. In: Biographical Memoirs, National Academy of Sciences Washington: National Academy Press 1-18
Nernst, Walther (1911). Zur Theorie der spezifischen Wärme und über die Anwendung der Lehre von den Energiequanten auf physikalisch-chemische Fragen überhaupt. Zeitschrift für Elektrochemie 17: 265-275
Phillips, Henry B. (1922). Review of . Bulletin of the American Mathematical Society 28: 69
Piaggio, Henry T. (1923). Review of . Mathematical Gazette 11: 276-278
Planck, Max (1988). The Theory of Heat Radiation. New York: American Institute of Physics.
- (1906). Vorlesungen über die Theorie der Wärmestrahlung. Leipzig: Johann Ambrosius Barth.
Prentis, Jeffrey J. (1995). Poincaré's Proof of the Quantum Discontinuity of Nature. American Journal of Physics 63: 339-350
Reiche, Fritz (1913a). Die Quantentheorie. Die Naturwissenschaften 1(23): 549-553
- (1913b). Gittererscheinungen auf verschiedenen Gebieten. Die Naturwissenschaften 1: 1193-1197
- (1914). Sieveking, H., Moderne Probleme der Physik. Die Naturwissenschaften 2: 887-888
- (1915). Valentiner, Siegfried, Die Grundlagen der Quantentheorie in elementarer Darstellung […]. Anwendung der Quantenhypothese in der kinetischen Theorie der festen Körper und der Gase. Die Naturwissenschaften 3: 248
- (1918). Die Quantentheorie. Ihr Ursprung und ihre Entwicklung.. Die Naturwissenschaften 6: 213-230
- (1921a). Die Quantentheorie. Ihr Ursprung und ihre Entwicklung. Berlin: Julius Springer.
- (1921b). Aus dem Weltbild der neuen Physik. Charlottenburg: Volkshochschulverlag.
Rhodes, Richard (1986). The Making of the Atomic Bomb. New York: SimonSchuster.
Richardson, Owen W. (1911). The Dynamical Effects of Aggregates of Electrons. Proceedings of the American Philosophical Society 50: 347-365
- (1914). The Electron Theory of Matter. Cambridge: Cambridge University Press.
Sarton, George (1921). Reiche, Fritz. Die Quantentheorie. Ihr Ursprung und ihre Entwicklung.. Isis 4: 375-376
Segrè, Emilio (1993). A Mind Always in Motion: The Autobiography of Emilio Segrè. Berkeley: University of California Press.
Sieveking, Hermann (1914). Moderne Probleme der Physik. Braunschweig: Friedrich Vieweg und Sohn.
Sieveking, Hermann, E. Viefhaus (1911). Über die Strahlungsgesetze, das Wirkungsquantum und das Nernstsche Theorem. Verhandlungen des naturwissenschaftlichen Vereins in Karlsruhe 25: 99-134
Sopka, Katherine R. (1988). Quantum Physics in America. New York: American Institute of Physics.
Stibbe, Matthew (2008). British Civilian Internees in Germany: The Ruhleben Camp, 1914–1918. Manchester: Manchester University Press.
Stoltzenberg, Dietrich (1994). Fritz Haber: Chemiker, Nobelpreisträger, Deutscher, Jude. Weinheim: VCH.
Stöltzner, Michael (2003) Causality, Realism and the Two Strands of Boltzmann's Legacy (1896-1936). phdthesis. University of Bielefeld
Stuewer, Roger H. (1971). William H. Bragg's Corpuscular Theory of X-Rays and γ-Rays.. British Journal for the History of Science 5: 258-281
- (1975). The Compton Effect: Turning Point in Physics. New York: Science History Publications.
Valentiner, Siegfried (1907). Vektoranalysis. Leipzig: G. J. Göschen'sche Verlagshandlung.
- (1914a). Die Grundlagen der Quantentheorie in elementarer Darstellung. Braunschweig: Friedrich Vieweg und Sohn.
- (1914b). Anwendung der Quantenhypothese in der kinetischen Theorie der festen Körper und der Gase. Braunschweig/Wiesbaden: Friedrich Vieweg und Sohn.
Wehefritz, Valentin (2002). Verwehte Spuren: Prof. Dr. phil. Fritz Reiche. Dortmund: Universitätsbibliothek.
Weinmeister, Paul (1926). J. C. Poggendorff's biographisch-literarisches Handwörterbuch für Mathematik, Astronomie, Physik, Chemie und verwandte Wissenschaftsgebiete. Leipzig: Verlag Chemie.
Wheaton, Bruce R. (1983). The Tiger and the Shark: Empirical Roots of Wave-Particle Duality. Cambridge: Cambridge University Press.
Yukawa, Hideki (1982). “Tabibito” (The Traveler). Singapore: World Scientific.
Footnotes
The word “textbook” encompasses a multitude of sins. Here I am including under that rubric books written for a professional audience. Since quantum theory was an advanced topic early in the twentieth century, the distinction between books intended specifically for students, and ones aimed at a more general professional audience, was at best hazy.
Remarkably, as of this writing (September 2010), Reiche’s book is still in print, in both the German original and the English translation. An electronic copy of the German edition may be found on Google books. The quotations used here draw on the English translation, but often revise it. Other translations in this chapter are my own.
For more comprehensive biographical sketches of Reiche, see (Bederson 2005; Wehefritz 2002). The latter includes a bibliography of Reiche’s publications.
See also Thomas S. Kuhn and George E. Uhlenbeck, interview with Fritz Reiche, March and April 1962, Archive for History of Quantum Physics (AHQP). See esp. session 2, pp. 1–2. This episode reminds us that in spite of exceptions like Planck and Einstein, it was still uncommon for physicists to restrict themselves exclusively to theory. It was therefore sensible for both Reiche and Born to seek some background in experimental work. Nevertheless, as their careers demonstrate, their lack of an experimental background was not an insuperable obstacle. See, for example, (Jungnickel and McCormmach 1986).
See Reiche’s description in his AHQP interview (footnote 4), session 1 (30 March 1962), p. 2, and session 2 (4 April 1962), p. 8.
Letter from Reiche to Kuhn, 17 July 1962, in the interview file (footnote 4).
For more on Berliner and Die Naturwissenschaften, see (Stöltzner 2003).
This conference, named for its sponsor, the Belgian industrialist Ernst Solvay, and organized by the physical chemist Walther Nernst, explored the implications of the new quantum theory and served to introduce it to a wider scientific audience. See for example (Kormos Barkan 1999, chap. 11).
Die Naturwissenschaften 2 (1914), 662–663; 4 (1916), 650; 9 (1921), 18.
The Eisenlohr foundation may be related to Wilhem Eisenlohr, who taught at the Karlsruhe Technische Hochschule in the mid-nineteenth century; see (Jungnickel and McCormmach 1986, vol. 2, 85). I have been unable to learn anything more about this prize or the foundation.
The PTR, as it is often called, was founded in 1887, and might best be described as a national laboratory concerned with integrating pure science with the technological needs of industry. As such, it served as a model for the National Physical Laboratory in England and the National Bureau of Standards (now the National Institute of Standards and Technology) in the United States, both founded a little over a decade later. See (Cahan 1989).
See also (Cahan 1989). Much of the experimental work on black-body radiation that contributed to Planck’s discovery of his radiation law late in 1900 was done at the PTR.
Valentiner served on several occasions as rector at Clausthal during the 1920s and 1930s. This history of the Bergakademie in these years does not seem to have been studied in great detail; but see (Müller 2005; 2007).
See (Weinmeister 1926) and later editions of Poggendorff.
Richardson had touched on Planck’s radiation law as early as 1911 in an essay in the Proceedings of the American Philosophical Society (Richardson 1911). Moreover, Richardson and one of his students at Princeton, Karl T. Compton, conducted experiments on the photoelectric effect; see (Stuewer 1975, 63–64).
For a discussion, see (Gearhart 2010).
Valentiner and Richardson mentioned the Solvay conference in their books. Sieveking did not, either in his book or his earlier article; he did, however, cite the well known paper of Henri Poincaré that appeared in the aftermath of that conference; see for example (Prentis 1995).
See also http://ruhleben.tripod.com/index.html, accessed 31 July 2012. Brose achieved the remarkable distinction of being interned twice: in Germany during the Great War, and in Australia during World War II. See (Jenkin 1993).
Reiche took a different tack in a little-known and considerably shorter (just under 50 pages) book, From the Worldview of the New Physics, (Reiche 1921b). This book, intended for a popular audience, included numerous drawings, but no equations and no bibliography or notes. It must have been among the earliest popular accounts of quantum theory.
Planck’s constant
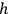 has units of “action”—the product of (generalized) momenta and coordinates. Planck’s and Sommerfeld’s treatments of action were different, but both involved less emphasis on quantized energies.
has units of “action”—the product of (generalized) momenta and coordinates. Planck’s and Sommerfeld’s treatments of action were different, but both involved less emphasis on quantized energies.
This striking phrase, first introduced in (Reiche 1913a), did not survive in the English translation.
Both the first edition (1906) in the original German and a translation of the second edition (1913) are reprinted in The Theory of Heat Radiation (Planck 1988). By 1913, Planck’s theory had changed substantially; the 1913 translation cannot be used as a guide to the 1906 edition.
Note that these favorable British reviews appeared during the early 1920s, when British and German scientists were often at loggerheads in the aftermath of the Great War.
See (Kuhn 1978, chap. X). Indeed, Planck’s “second theory” did slowly fall out of favor during the late teens and early 1920s.
In a section not quoted by Stanley, Bragg added, “Toleration of opinions is a recognized virtue. The curiosity of the present situation is that opposite opinions have to be held and used by the same individual in the faith that some day their combined truth may be made plain.” Bragg was writing in the context of his experimental and theoretical efforts to understand X-rays; see (Stuewer 1971; 1975; Wheaton 1983).
Richardson (1914, 507–508), took a similar point of view, even though he was skeptical of Einstein’s light quanta; see (Knudsen 2001, 244–245).
Phillips was a mathematician at the MIT. Like several other reviews of Reiche’s book, this one appeared in a mathematics journal.
See for example (Sopka 1988, 91–95 and 175–193), for the United States.
See for example the lecture notes of Peter Debye at Göttingen (circa 1914) and Edwin C. Kemble at Harvard (early 1920s), AHQP, reels 24, 55–57. See also the chapter by Midwinter and Janssen in this volume.
For more on Briggs, including lists of publications, see (Myers and Levelt Sengers 1999; Landa and Nimmo 2003).
